Introduction

Cement is one of the most important inventions in construction. From the walls of our homes to the massive bridges and smooth highways, cement is almost everywhere. It is the binding material that binds sand, gravel, and water together and giving strength and durability to the concrete.
The idea of using binding materials to join stones and bricks is not new today. Ancient builders also used lime and volcanic ash for building temples and aqueducts, but modern cement has transformed construction into what we see today, i.e. faster, stronger, and more reliable.
In this article, we will discuss what is cement. history, properties, and the physical & chemical composition of cement. Whether you are a student preparing for exams or an engineer working on-site, understanding cement is essential for building strong and durable structures.
What is Cement?
Cement is a fine powder substance that works as a binder for construction materials. When it is mixed with water, it reacts chemically and forms a paste that sets and hardens over time. After the hydration process, it binds sand, gravel together to create concrete and mortar.
The word cement comes from the Latin word caementum, which means “building stone”. Modern cement, known as Portland cement, was invented in 1824 by Joseph Aspdin.
Cement has the unique property of setting and gaining strength even underwater. So it is ideal for building not only houses and road pavements but also bridges, dams, tunnels, and massive structures.
In simple words, we can say that cement is the key ingredient that transforms a loose mixture of sand and gravel into strong, durable concrete. Without it, most of the world’s modern infrastructure, from skyscrapers to highways, would not exist.
History of Cement
The idea of using a binding material to bind stones and bricks together is thousands of years old. Centuries later, the Romans invented mixing lime with volcanic ash, known as pozzolana, which produced a strong mortar that could even harden underwater. This invention allowed them to build aqueducts, bridges, and domes that survived for more than two thousand years.
According to wikipedia Modern cement was developed much later. In 1824, an English bricklayer named Joseph Aspdin invented a new type of cement by mixing limestone and clay. He called it Portland Cement because its hardened appearance is similar to the natural limestone found on the island of Portland in England. This is the beginning of the cement we use today.
By the late nineteenth century, the introduction of rotary kilns and better manufacturing techniques made it possible to produce cement on a large scale with consistent quality.
In India, the first cement factory was set up in 1904 at Chennai, which is the foundation for the country’s cement industry. Over time, various types such as Ordinary Portland Cement (OPC), Portland Pozzolana Cement (PPC), and blended cements were developed to meet the demands of the modern construction industry.
These improvements have made cement stronger, more durable, and more suitable for sustainable infrastructure.
Raw Materials of Cement

Cement is made by mixing lime-rich Calcareous materials and clay-rich argillaceous materials in the right proportion, usually 3:1, and then heating them at a very high temperature in a rotary kiln. The quality and proportion of these raw materials decide how strong, durable, and stable the cement will be.
Calcareous Materials: Lime-Rich
Calcareous materials provide lime (CaO), which is up to 60–65% in cement. The most common sources of Calcareous materials are limestone, chalk, and sometimes marine shells, especially in coastal areas.
Lime is the main ingredient that reacts with silica, alumina, and iron oxide during burning in the kiln to form the binding compounds of cement. However, if there is too much lime, the cement becomes unsound and may crack after it sets.
Argillaceous Materials: Clay-Rich
Argillaceous materials supply silica (SiO₂) and alumina (Al₂O₃). Common sources of argillaceous materials are clay, shale, slate, and sometimes blast-furnace slag, which is an industrial by-product used in the manufacturing of cements.
Silica and alumina react with lime during burning to form bouge’s compounds such as C₃S, C₂S, and C₃A, which provide the cement strength and durability.
Corrective Materials
To balance the composition, small amounts of other materials are added, such as:
- Iron oxide- It helps in fusing the mix and gives cement its color.
- Magnesia – It improves the hardness of cement, but must be kept in control to prevent expansion and cracking.
- Sulphur compounds help in regulating the setting time.
- Gypsum- It is added at the final grinding stage to slow down the setting time and make the cement workable.
Properties of Cement
The performance of cement in concrete or mortar depends on its properties. These properties determine how cement behaves during mixing, setting, and hardening of cement. A good quality cement ensures the strength and durability of structures.
The properties of cement are mainly divided into physical properties and chemical properties, which are explained below.
Physical Properties of Cement
The physical properties describe how cement looks and behaves during construction.
Fineness
Cement is ground into a very fine powder. The finer the cement, the larger the surface area available for reaction with water, which helps in developing strength faster. However, if the cement is too fine, it may require more water and can shrink after hardening.
Specific Gravity
The specific gravity of Ordinary Portland Cement (OPC) is about 3.15. This property helps engineers calculate the correct mix proportions for concrete.
Bulk Density
The bulk density of loose cement is about 1440 kg/m³. It is important to know this for storage and transportation.
Standard Weight and Volume
Cement is usually packed in bags weighing 50 kg, each bag occupying a volume of about 0.035 m³.
Setting Time
Cement does not harden immediately after adding water. The initial setting time is about 30 minutes, which allows enough time for mixing and placing. The final setting time is about 10 hours, after which the cement paste becomes hard and stable. So to control this setting time and avoid quick hardening (flash setting), about 3–5% gypsum is added.
Soundness
Soundness means that the cement does not expand after setting. If cement contains excess lime or magnesia, it may expand later and lead to cracks. A sound cement keeps the structure stable and durable.
Heat of Hydration
When cement reacts with water, it releases heat. In small works, this heat does not create a problem because proper cooling is done, but in massive structures like dams, it can cause cracks due to uneven cooling. So, low-heat cement is often used in such a massive structure.
Chemical Properties of Cement
Chemical properties relate to how cement reacts with water and the environment.
Hydraulic Nature
Cement can set and harden even underwater because it reacts with water to form strong binding compounds.
Composition Stability
The correct proportion of lime, silica, alumina, and other oxides is important. Too much lime and magnesia can make cement unsound, while too much silica can slow down the setting process. So a balanced composition ensures both early and long-term strength.
Resistance to Chemical Attack
Some special cements are designed to resist damage caused by chemicals present in soil or water, such as sulphates and chlorides. This helps in building durable structures in aggressive environments.
Chemical Composition of Cement
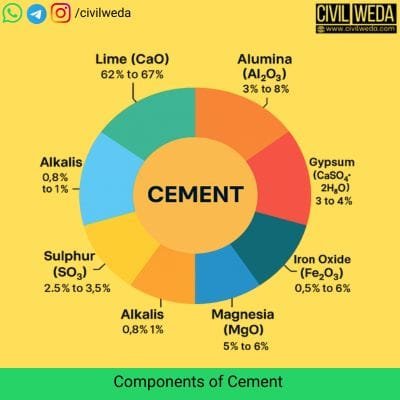
Cement contains several chemical compounds that control its strength, durability, setting time, and heat of hydration. These compounds are formed when a mixture of calcareous materials and argillaceous materials is heated in a rotary kiln to a temperature of about 1450°C and then ground with a small amount of gypsum.
The table below shows the main compounds of cement, their proportion, and their role in construction.
| Ingredient | Composition % by Weight | Function in cement |
| Lime (CaO) | 60 – 65% | The main component of cement. It reacts with silica and alumina to form hard compounds that give strength. |
| Silica (SiO₂) | 17 – 25% | Makes the cement harder and improves its long-term strength. |
| Alumina (Al₂O₃) | 3 – 8% | Lowers the burning temperature and helps the cement to set faster. |
| Iron Oxide (Fe₂O₃) | 0.5 – 6% | It helps in the chemical reaction and strength. It also gives the typical grey colour to the cement. |
| Magnesia (MgO) | 0.5 – 4% | Improves strength in small amounts but can cause cracks if it’s too high. |
| Sulphur Trioxide (SO₃) | 1 – 3% | Comes partly from gypsum; it helps in regulating the setting time. |
| Gypsum (CaSO₄·2H₂O) | 3 – 5% | Added during the final grinding stage to slow down the setting time and prevent flash-setting of cement. |
Hydration of Cement
When water is added to cement, a chemical reaction starts. This reaction is called the hydration of cement. During hydration, the main compounds of cement, especially C₃S and C₂S, react with water and form new products. These products gradually make the cement paste hard and strong.
The most important product of this reaction is Calcium Silicate Hydrate (C-S-H gel), which is responsible for binding the particles of sand and gravel together and giving concrete its strength. Another product, Calcium Hydroxide [Ca(OH)₂], is also formed, but it does not contribute much to strength.
This reaction also releases heat, known as the heat of hydration. In massive concrete structures like dams, the heat generated during the hydration of cement can be so high. It causes cracks if it is not controlled properly. So engineers often use low-heat cement and proper curing techniques to keep the concrete moist and cool for these massive structures.
Uses of Cement in Construction
Cement is the most important material in modern construction. Its ability to bind sand, gravel, and water into a hard, durable mass makes it essential for almost every type of structure, from small houses to massive bridges and dams. It is used in Concrete Production, making Mortar for Masonry Work, Plastering and Finishing, Precast, Grouting and Waterproofing, Repair and Maintenance, etc.
Read more Civil Engg Topics
Conclusion
Cement is the backbone of modern construction. Its unique ability to bind sand, gravel, and water into a strong and durable mass has made it the most important material for building everything from small houses to massive dams and bridges.
Understanding what is cement, how it was developed, and how its properties, chemical composition, and hydration process affect strength and durability is essential for both students and engineers.
This knowledge helps in choosing the right type of cement for each project and ensures that the structures built are safe, strong, and long-lasting.
As construction technology advances, new types of cement, such as blended cements and geopolymer cement, are offering more sustainable and durable options.
By learning the basics of cement, future engineers can make better decisions on site and contribute to building infrastructure that stands the test of time.
FAQs on Cement
What is cement in simple words?
Cement is a fine powder that acts as a binding material in construction. When mixed with water, it reacts chemically, binding sand and gravel together to form strong concrete and mortar as required.
Who invented modern cement?
Modern cement was invented by Joseph Aspdin in 1824. He named it Portland because the hardened material looked like the natural Portland stone found in England.
What are the main raw materials used to make cement?
Cement is mainly made from two types of raw materials:
1. Calcareous materials (lime-rich) such as limestone and chalk
2. Argillaceous materials (clay-rich) such as clay, shale, or slate
What is the specific gravity of cement?
The specific gravity of Ordinary Portland Cement (OPC) is about 3.15.
Why is gypsum added to cement?
About 3–5% gypsum is added during the grinding of clinker to control the setting time. Without gypsum, cement would set too quickly (flash setting), making it difficult to mix and place properly.
Which compound in cement gives early strength?
The compound Tricalcium Silicate (C₃S) is mainly responsible for early strength in cement. It reacts quickly with water and helps the concrete harden in the first few days.
What is the heat of hydration of cement?
The heat of hydration is the heat released when cement reacts with water. This heat is higher in OPC because it reacts faster. In large concrete structures like dams, too much heat can cause cracks, so low-heat cement is often used.
Thank You for Reading! 🙏
We hope this article helped you clearly understand what cement is in civil engineering. If you found this complete article useful, please share it with your friends and university students. For more informative posts on civil engineering topics, stay connected with Civil Weda. 🚀


