Introduction

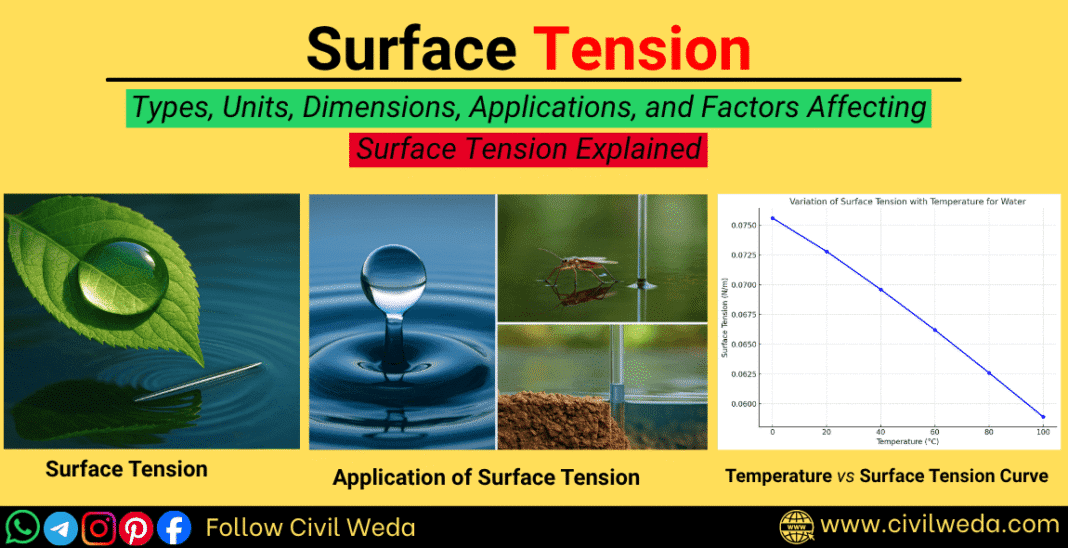
When you see small water drops resting on a leaf or glass surface, have you ever wondered why they are always round in shape instead of flat? This simple observation reveals a remarkable property of liquids known as surface tension.
In fluid mechanics, surface tension is a concept that explains how and why the surface of a liquid behaves like a stretched elastic film. It is the invisible force that keeps water drops together and helps small insects walk on water.
For civil engineers, understanding surface tension is essential. It plays a major role in capillary action and soil moisture movement. In this article, we’ll explore why surface tension actually occurs, factors affecting it, and how it influences real-world engineering applications.
Definition of Surface Tension
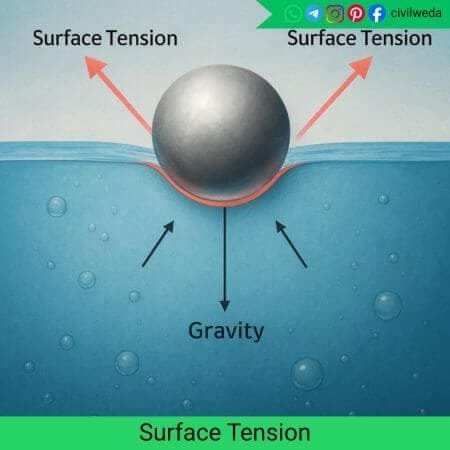
In simple terms, surface tension is defined as the property of a liquid surface that causes it to behave like a stretched elastic sheet. It resists any force that tries to increase its surface area.
It is the tensile force acting along the surface, due to which the surface molecules are pulled inward. This inward pull makes the liquid surface contract and take the smallest possible area.
Scientifically, it may be defined as:
“The force per unit length acting on the surface of a liquid in contact with a gas (or another immiscible liquid), which tends to minimize the surface area of the liquid.”
Mathematically
If a force F acts along a liquid surface of length L, then
Surface Tension = F / L
where,
T = (Surface Tension) (N/m)
F = Force acting along the surface (N)
L = Length on which the force acts (m)
Units and Dimensions of Surface Tension
It is a force per unit length, so its unit and dimension are as given below:
- SI Unit: Newton per meter (N/m)
- CGS Unit: Dyne per centimeter (dyn/cm)
- Dimensional Formula: (M¹T⁻²)
Why Surface Tension Occurs
To understand why surface tension occurs, we have to look at what happens at the molecular level of a liquid.
Every liquid is made up of countless tiny molecules; these molecules are always attracting each other through cohesive forces.
Inside the liquid, each molecule is surrounded by other molecules in all directions, so the attractive forces are balanced to each other; there is no net force on those molecules, as shown in the figure.
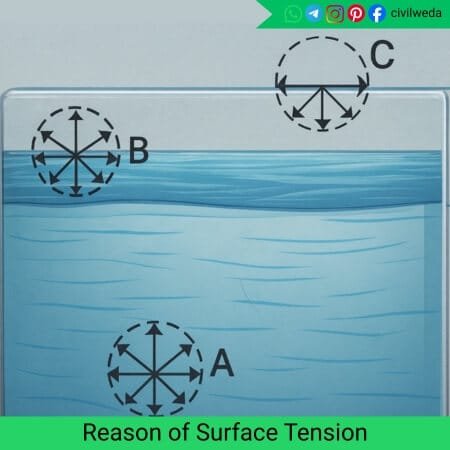
However, the situation is different for the molecules present on the surface. These surface molecules do not have other liquid molecules above them; only air is present. Because of this, they experience a net inward pull from the molecules.
This inward pull makes the surface behave like a stretched elastic film, just like a stretched elastic sheet. Due to this tension, the liquid always tries to reduce its surface area and stay in the smallest possible shape.
That’s why droplets of liquid tend to form spherical shapes, since a sphere has the smallest possible surface area for a given volume.
Note: It depends on cohesive forces. When cohesive forces increase, surface tension also increases, and vice versa.
I hope you now clearly understand why surface tension occurs at the molecular level. Now, let us understand the factors that affect it.
Measurement of Surface Tension
It cannot be measured directly, but it can be determined using experimental methods. Several practical methods are available to measure the surface tension of a liquid. The most commonly used methods are the Capillary Rise Method, the Drop Weight Method, or Drop Volume Method, the Maximum Bubble Pressure Method, etc.
Among these, the capillary rise method is the simplest and most common method, in which the height of liquid rise in a thin glass tube is used to calculate surface tension. If you want to learn about these methods in detail, including formulas, diagrams, and practical examples, you can read our separate post.
Factors Affecting Surface Tension
The surface tension of a liquid does not remain the same under all conditions. It changes when the temperature, purity, or nature of the liquid changes. The main factors that affect it are explained below.
1. Temperature
When the temperature of a liquid increases then the kinetic energy of the liquid molecules also increases. When the kinetic energy of the liquid increases, the cohesive forces between the molecules of the liquid decrease, so the surface tension decreases.
In simple words, surface tension decreases with the increase in temperature. A graphical representation of surface tension vs temperature is shown in the figure:
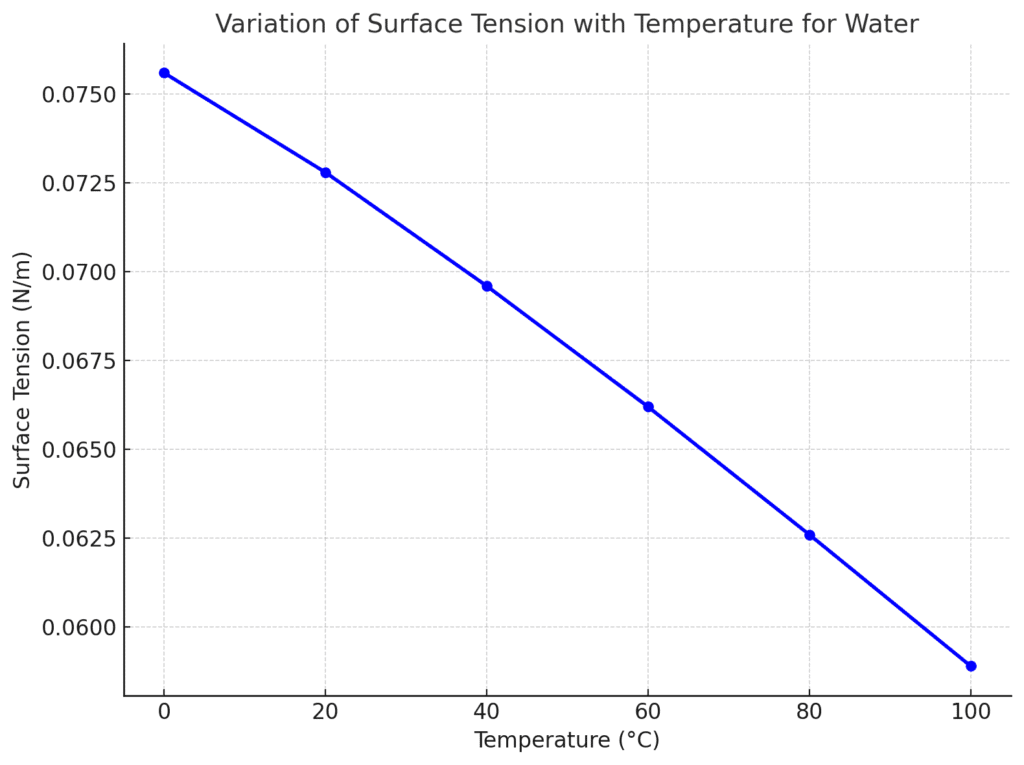
For Example, Hot water has lower surface tension than cold water, which is why hot water spreads more easily than cold water.
2. Impurities
The presence of impurities in a liquid can either increase or decrease its surface tension, depending on the type of impurity.
If the impurity reduces cohesive forces (like soap or detergent in water), the surface tension decreases. And, if the impurity increases cohesive forces (like salt or some acids), the surface tension increases.
Example: Adding soap or detergent to water lowers its surface tension and spreads on cloth, helping it clean better. And when adding soap or detergent to hot water, its surface tension decreases more. So, cloth cleaning in hot water is easier than in cold water.
3. Nature of the Liquid
As I explained earlier, Surface tension depends on cohesive forces. When cohesive forces increase, it also increases, and vice versa.
So, different liquids have different types of cohesive forces (molecular forces). Liquids with strong cohesive forces, like mercury, have high surface tension. Those with weak cohesive forces, like alcohol, have low surface tension.
Example: Mercury forms almost perfect spherical drops, whereas alcohol spreads easily on the surface.
I hope you now clearly understand the factors affecting surface tension. Now, let’s explore some common applications of surface tension in our daily life.
Examples and Applications of Surface Tension

This can be easily observed in our daily lives. It plays an important role not only in nature but also in many engineering and industrial processes. Some common examples and applications of surface tension are given below:
1. Formation of Water Droplets
When water falls from a tap or condenses on a surface, it forms small, round droplets. This happens because surface tension pulls the water molecules together, making the droplet take a spherical shape, which has the smallest surface area for a given volume.
2. Capillary Action
This is one of the main reasons behind capillary rise, the upward movement of a liquid in narrow tubes or small pores. This phenomenon is important in soils, concrete pores, and building materials, as it controls the movement of water within them.
3. Floating of Small Objects
Small and light objects like needles, blades, or insects can float on the water surface, even though they are denser than water. This is because surface tension creates a thin elastic film on the surface that supports them gently.
4. Action of Detergents and Soaps
Detergents and soaps reduce the surface tension of water, helping it spread and wet the surface more effectively. This allows water to enter the tiny spaces in cloth, improving cleaning or wetting efficiency.
5. Waterproofing and Coating Applications
In civil engineering, bitumen coating, paints, and chemical waterproofing compounds depend on surface tension.
6. Curing of Concrete
During concrete curing, it affects the rate of water evaporation. High surface tension slows down evaporation, while low surface tension allows moisture to escape quickly. Controlling this helps in maintaining proper strength and hydration in the concrete surface.
Importance of Surface Tension in Civil Engineering
Surface tension is not directly important, but indirectly it plays an important role in many civil engineering applications, especially where liquids interact with solid materials such as soil, concrete, paint, and bitumen. In soil, it is responsible for capillary rise, which helps water move upward through small pores. This phenomenon is essential for understanding groundwater movement, soil moisture distribution, and foundation design. However, excessive capillary rise can also cause dampness in walls and structures, so it must be properly controlled during construction.
In waterproofing and bitumen coating works, surface tension helps the coating spread uniformly and adhere well to the surface. Proper control of surface tension ensures good waterproofing and protection of structures. Similarly, in paints and surface finishes. It allows the paint to spread smoothly over walls and other surfaces. If it is too high, the paint does not spread properly; if it is too low, it may flow unevenly.
During concrete curing, it helps retain moisture on the surface, which is essential for proper hydration of cement and the development of strength. It also affects the wetting and bonding of construction materials like mortar and plaster, improving their workability and adhesion. Thus, surface tension, though a microscopic property, has a significant impact on the quality, durability, and performance of construction materials in civil engineering.
Read more Civil Engg Topics
- Viscosity
- Portland Cement
- Bitumen Waterproofing
- Instruments used in a chain survey
- Bulking of sand
- Bitumen concrete
Conclusion
Surface tension is a fundamental property of fluids that explains many natural and engineering phenomena. It results from the cohesive forces between liquid molecules, which make the surface behave like a stretched elastic film. Factors such as temperature, impurities, and the nature of the liquid influence its value.
In civil engineering, it is essential for analyzing capillary rise in soils, the behavior of bitumen coatings, waterproofing materials, and the curing of concrete. Though it acts on a microscopic level, its effects are clearly visible in large-scale structures and construction processes.
In short, surface tension connects basic fluid mechanics with practical applications in civil engineering — helping engineers design materials and structures that perform efficiently and last longer.
FAQs on Surface Tension
1. Who discovered the surface tension force?
The concept of surface tension was first studied and explained by Thomas Young in the early 19th century. Later, it was mathematically developed by Pierre-Simon Laplace
2. What is the unit and dimension of surface tension?
Its unit and dimensions are-
SI Unit: Newton per meter (N/m)
Dimension formula:(M¹T⁻²)
3. Does temperature affect surface tension?
Yes. As temperature increases, the cohesive forces between liquid molecules become weaker, and surface tension decreases.
4. What causes surface tension in liquids?
Surface tension occurs due to the unbalanced cohesive forces acting on the molecules at the surface of a liquid. The molecules at the surface are pulled inward, creating tension.
5. What is the surface tension of water?
The surface tension of pure water at 20°C is about 0.0728 N/m (or 72.8 × 10⁻³ N/m). It decreases as temperature increases because heat weakens the hydrogen bonding between water molecules. For example, at 100°C, the surface tension of water drops to around 0.0589 N/m.
Thank You for Reading! 🙏
We hope this article helped you clearly understand the surface tension in civil engineering. If you found this complete article useful, please share it with your friends and university students. For more informative posts on civil engineering topics, stay connected with Civilweda. 🚀