Introduction to Chlorination of Water

Safe drinking water is one of the most essential needs for human survival. However, natural water sources often contain harmful microorganisms such as bacteria, viruses, and parasites that can cause serious diseases. To make water safe and suitable for consumption, disinfection methods are applied, and among them, chlorination of water is the most widely used and effective technique.
Chlorination refers to the process of adding chlorine or chlorine-based compounds to water to kill pathogens and improve overall water quality. It is not only cost-effective but also ensures residual protection, meaning it continues to safeguard water from recontamination during storage and distribution. Because of its reliability and simplicity, chlorination has become a standard method in municipal water supply systems and treatment plants across the world.
Definition of Chlorination of Water
Chlorination of water is a chemical process in which chlorine or chlorine-based compounds are added to water for the purpose of disinfection. The primary aim is to kill harmful microorganisms such as bacteria, viruses, and parasites, thereby making the water safe for drinking and domestic use.
When chlorine dissolves in water, it undergoes a reaction to form hypochlorous acid (HOCl), which is the most powerful disinfecting agent. This compound penetrates the cell walls of pathogens and destroys them effectively. The reaction can be represented as:
Cl₂ + H₂O → HOCl + HCl
- HCl (Hydrochloric Acid): A by-product of the reaction.
- HOCl (Hypochlorous Acid): The main disinfectant responsible for killing microorganisms.
In simple words, chlorination is one of the most reliable and economical methods to ensure that water remains safe not only at the treatment stage but also throughout storage and distribution.
Objectives of Chlorination of Water
The primary purpose of chlorination of water is to make it safe, clean, and suitable for human consumption as well as domestic use. Since raw water often contains harmful organisms and impurities, chlorination serves multiple functions. There are some objectives of chlorination of water are explained below:
1. Destruction of Pathogenic Microorganisms
The main objective is to eliminate disease-causing organisms such as bacteria, viruses, and protozoa. These microorganisms are responsible for water-borne diseases like cholera, typhoid, dysentery, blue baby syndrome, and hepatitis etc. Chlorine effectively penetrates their cell walls and destroys their internal structure, thereby preventing the spread of epidemics.
2. Improvement of Taste, Odor, and Appearance
Natural water sources may sometimes have unpleasant taste, foul odor, or visible coloration due to the presence of organic matter, algae, or industrial pollutants. Chlorination helps in reducing these undesirable characteristics, thus improving the overall acceptability of water for drinking purposes.
3. Provision of Residual Protection
One of the unique advantages of chlorination is that it provides a residual effect. This means that a small quantity of chlorine remains in the water even after treatment. This residual chlorine acts as a protective shield, preventing recontamination during storage in tanks, pipelines, or household containers.
4. Control of Algae, Slime, and Biofilm Growth
In distribution systems, pipelines, and storage tanks, algae and slime layers often develop, which reduce water quality and clog the system. Chlorination helps in controlling such biological growth and ensures smooth water flow without contamination.
5. Oxidation of Dissolved Impurities
Apart from disinfection, chlorine also oxidizes dissolved impurities such as iron, manganese, and hydrogen sulfide. These substances cause staining of clothes, a bad odor, and corrosion in pipes. Chlorination converts them into insoluble forms, which can be removed by subsequent treatment processes.
Types of Chlorination of Water
Depending on when and how chlorine is applied, the chlorination of water is classified into several types. Each method has its own importance, benefits, and limitations.
1. Plain Chlorination of Water
- In this method, only chlorine is added to the water without any pre-treatment like coagulation, sedimentation, or filtration.
- It is effective only when the raw water is relatively clean, colorless, and has low turbidity.
- Mostly used in rural or emergency water supply systems where water is drawn from protected sources such as deep wells.
- Advantages:
- Very simple and economical.
- Quick application in areas lacking advanced treatment plants.
- Limitations:
- Not suitable for highly polluted or muddy water.
- Less effective if organic matter is present in excess.
2. Pre-Chlorination of Water
- In this method, chlorine is added at the initial stage of treatment, usually before sedimentation or filtration.
- Purpose:
- To control biological growth, such as algae and slime.
- To reduce odor and taste.
- To oxidize dissolved iron and manganese so that they can be easily removed.
- Advantages:
- Enhances the performance of sedimentation and filtration units.
- Reduces the chances of clogging in filters.
- Limitation: May form more disinfection by-products (DBPs) if water contains organic matter.
3. Post-Chlorination of Water
- This is the most common and widely used method of chlorination.
- Here, chlorine is added at the last stage of treatment, just before the treated water enters the distribution system.
- Purpose:
- To ensure that safe water is supplied to the consumers.
- To provide residual chlorine in the water for protection against recontamination.
- Advantage: Ensures continuous protection in the supply pipelines and storage tanks.
4. Double Chlorination of Water
- In this method, chlorine is applied twice – first as pre-chlorination and later as post-chlorination.
- Purpose:
- To provide extra protection when the raw water is highly contaminated.
- To safeguard water during long distribution networks where the chances of contamination are higher.
- Applications: Emergency situations, rural supplies, or in areas where water quality is doubtful.
5. Breakpoint Chlorination
- This is one of the most scientifically accurate methods of chlorination.
- When chlorine is added to water containing impurities and ammonia, it first reacts to form chloramines.
- Initially, no free chlorine is available, but as more chlorine is added, a stage is reached where all chlorine-demanding substances are completely oxidized.
- The point beyond which further chlorine addition results in free residual chlorine is called the breakpoint.
- Advantages:
- Ensures complete removal of ammonia and organic matter.
- Provides safe residual chlorine for long-term protection.
- Importance: Commonly used in municipal water treatment plants.
6. Super Chlorination of Water
- In this method, a very high dose of chlorine is added to water.
- Purpose:
- To deal with emergency situations such as outbreaks of cholera or typhoid.
- To disinfect water that is heavily polluted or unsafe.
- Since excess chlorine is harmful, a process called dechlorination (using chemicals like sodium thiosulfate or activated carbon) is carried out to remove extra chlorine from the water supply.
- Applications: Swimming pools, military camps, and areas affected by epidemics.
Process of Chlorination of Water
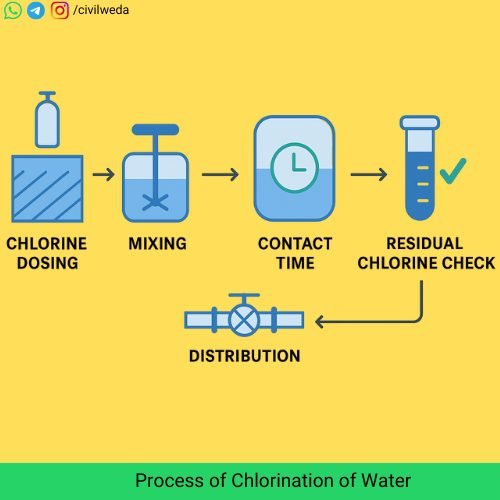
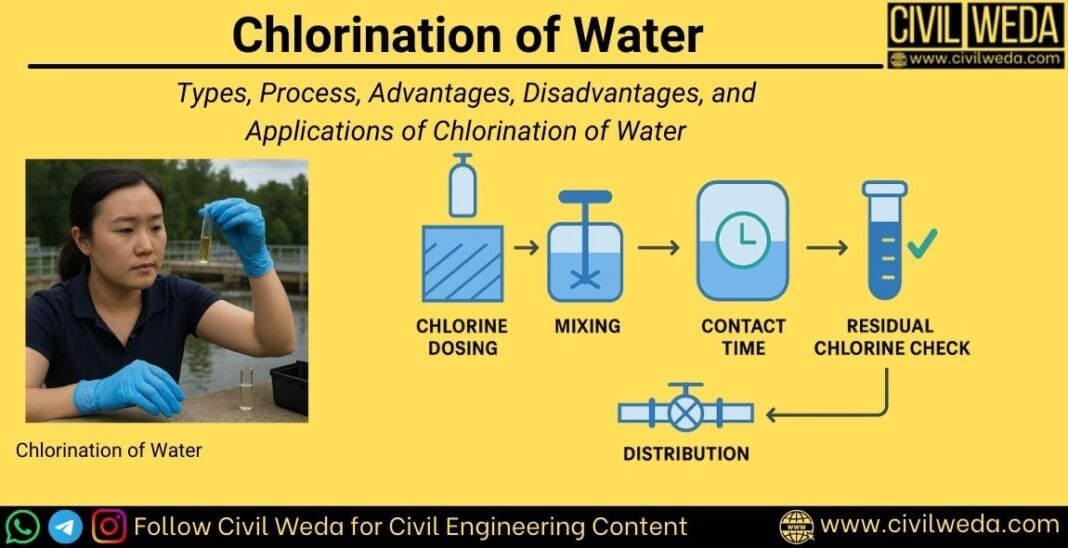
The process of chlorination of water is not just about adding chlorine, but about ensuring safe, reliable, and effective disinfection. It generally follows these steps:
1. Chlorine Dosing
- Chlorine can be applied in different forms, such as chlorine gas (Cl₂), sodium hypochlorite (NaOCl), calcium hypochlorite [Ca(OCl)₂], or bleaching powder.
- The exact dose depends on:
- Quality of raw water
- Level of contamination
- Presence of organic matter and ammonia
- For drinking water, the usual chlorine dose ranges between 1 to 5 mg/L.
2. Mixing of Chlorine with Water
- Proper mixing is very important; otherwise, some portions of water may remain untreated.
- Mixing can be done by:
- Mechanical mixers (paddle wheels, stirrers)
- Hydraulic mixing (through baffles or turbulence in chambers)
- Diffusion methods (bubbling chlorine gas directly in water)
3. Contact Time
- After dosing and mixing, chlorine needs time to act on pathogens.
- This period is called contact time, and water is usually kept in a contact tank.
- WHO recommends 30 minutes contact time at pH < 8.0 and residual chlorine of at least 0.5 mg/L.
4. Residual Chlorine Monitoring
- The purpose of chlorination is not just immediate disinfection, but also long-term safety.
- A small quantity of chlorine should remain in the water, known as residual chlorine.
- Ideal range: 0.2 – 0.5 mg/L at the consumer end.
- Too low → risk of contamination.
- Too high → causes bad taste, odor, and health issues.
5. Storage and Distribution
- Finally, chlorinated water is stored in reservoirs or supplied through pipelines.
- Continuous monitoring is done to ensure chlorine levels are safe and effective.
Factors Affecting Chlorination of Water
The success of chlorination depends on various physical, chemical, and biological factors:
1. pH of Water
- Chlorine reacts with water to form HOCl (Hypochlorous Acid) and OCl⁻ (Hypochlorite Ion).
- HOCl is 80–100 times more effective as a disinfectant than OCl⁻.
- At low pH (6–7) → more HOCl present → stronger disinfection.
- At high pH (>8) → more OCl⁻ present → weaker disinfection.
2. Temperature of Water
- Disinfection is faster in warm water because chemical reactions occur more rapidly.
- In cold water, the process is slower, and a longer contact time or higher dose is required.
3. Contact Time
- The effectiveness of chlorination is directly proportional to the contact time.
- Formula often used: CT Concept (C × T = concentration × time)
- Example: 1 mg/L chlorine for 30 minutes contact time = 30 mg·min/L CT value.
- Longer contact time ensures thorough disinfection.
4. Turbidity of Water
- High turbidity means suspended particles shield microorganisms from chlorine.
- Chlorine first reacts with organic matter and impurities before disinfecting pathogens.
- Therefore, turbid water requires a higher chlorine dosage.
5. Chlorine Demand of Water
- Different water sources have different chlorine demands, depending on ammonia, organic matter, and other impurities present.
- Chlorine must first satisfy this demand before residual chlorine appears.
- Example: If chlorine demand = 2 mg/L, and we add 3 mg/L, then residual chlorine = 1 mg/L.
6. Type of Microorganisms
- Some microorganisms (like Cryptosporidium and Giardia) are resistant to chlorine.
- Viruses are killed more easily, while cysts and spores may require higher doses or combined treatment methods (e.g., chlorination + filtration or UV).
7. Presence of By-Products
- When chlorine reacts with natural organic matter, harmful by-products such as Trihalomethanes (THMs) may form.
- This risk must be minimized by proper dosing and pre-treatment.
Advantages of Chlorination of Water
The chlorination of water is the most widely used disinfection method because of its simplicity, low cost, and effectiveness. Its major advantages are as follows:
1. Highly Effective Against Pathogens
Chlorine is powerful in destroying a wide range of disease-causing microorganisms, including bacteria and viruses. It reduces the risk of water-borne diseases like cholera, typhoid, dysentery, and hepatitis.
2. Provides Residual Protection
Unlike many other disinfection methods, chlorine leaves behind a residual effect in the water. A small quantity of chlorine (0.2–0.5 mg/L) remains even after treatment, which protects the water from recontamination during storage and distribution.
3. Economical and Easily Available
Chlorine is inexpensive compared to other disinfectants such as ozone or UV radiation. Its availability in different forms (chlorine gas, bleaching powder, sodium hypochlorite, calcium hypochlorite) makes it suitable for both urban and rural water supply systems.
4. Easy to Apply and Control
The process of chlorination is simple and does not require highly skilled manpower. With proper dosing equipment, chlorine can be applied and adjusted according to water quality.
5. Improves Taste and Odor
Chlorine helps to reduce foul odor and improve the taste of water by oxidizing organic impurities, hydrogen sulfide, and other undesirable substances.
6. Controls Biological Growth in Pipelines
Chlorination prevents the growth of algae, slime, and biofilms in pipelines, storage tanks, and distribution systems. This ensures a smooth flow of water without contamination.
7. Oxidizes Dissolved Impurities
Chlorine helps in oxidizing dissolved substances like iron (Fe²⁺), manganese (Mn²⁺), and hydrogen sulfide (H₂S), which otherwise cause staining, bad smell, and corrosion.
8. Versatile Applications
Chlorination is not limited to drinking water; it is also used in swimming pools, sewage treatment, industrial cooling systems, and emergency water disinfection.
Disadvantages of Chlorination of Water
Although chlorination is one of the most popular and effective methods of water disinfection, it also has certain drawbacks that need to be considered there Some disadvantages of chlorination of water are given below:
1. Formation of Harmful By-Products
- When chlorine reacts with natural organic matter present in water, it may form disinfection by-products (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs).
- Long-term exposure to these compounds is associated with health risks, including cancer and liver problems.
2. Unpleasant Taste and Odor
- Excess chlorine can leave a strong taste and odor in water, making it less palatable for drinking.
- Consumers often complain of a “swimming pool” like smell in over-chlorinated water.
3. Corrosive Nature
- Chlorine is corrosive and can damage metallic pipelines, storage tanks, and distribution systems if applied in high concentrations.
- This may lead to leakage, rusting, and increased maintenance costs.
4. Limited Effectiveness Against Some Microorganisms
- While chlorine is highly effective against bacteria and viruses, it is less effective against protozoan cysts such as Cryptosporidium and Giardia.
- These organisms require additional treatment methods like filtration or UV disinfection.
5. Safety Concerns in Handling
- Chlorine gas is toxic and hazardous to handle.
- Special care, trained manpower, and safety equipment are required to prevent accidents during storage and application.
6. Reduced Efficiency at High pH
- Chlorine works best at a pH range of 6.5–7.5.
- At higher pH levels, the disinfecting power decreases significantly due to the formation of less active hypochlorite ions (OCl⁻).
7. Sensitive to Turbidity and Impurities
- In turbid or highly polluted water, chlorine first reacts with organic matter, ammonia, and suspended solids.
- This increases chlorine demand and reduces its efficiency for pathogen destruction.
Applications of Chlorination of Water
Chlorination is not limited to drinking water disinfection; it has multiple applications in different sectors because of its effectiveness, low cost, and residual protection. The major applications of Chlorination of Water are:
1. Drinking Water Treatment
- The most common use of Chlorination of Water is in municipal water treatment plants.
- Chlorine ensures that large volumes of water supplied to the public are safe and free from disease-causing microorganisms.
- It is the primary line of defense against outbreaks of water-borne diseases such as cholera and typhoid.
2. Municipal Water Supply and Distribution
- After treatment, chlorine is applied (post-chlorination) to maintain a residual effect during storage and distribution.
- This residual chlorine prevents recontamination in pipelines, storage tanks, and consumer taps.
3. Swimming Pools
- Chlorine is extensively used to disinfect swimming pools.
- It prevents the spread of infections through water and controls the growth of algae and slime in the pool.
4. Industrial Applications
- Industries use chlorination for cooling water treatment, controlling biological growth in pipelines, and preventing fouling of equipment.
- It is also applied in food processing industries to ensure a clean water supply.
5. Sewage and Wastewater Treatment
- Chlorine is used in sewage treatment plants to disinfect effluents before releasing them into rivers or lakes.
- This reduces the risk of contaminating natural water bodies and protects aquatic life as well as human health.
The applications of chlorination of water range from household water safety to municipal, industrial, and emergency uses, making it one of the most versatile disinfection methods in the world.
Read more Civil Engg topics
- Chlorination of water
- Per Capita Water Demand
- Pile foundation
- Drip Irrigation
- Instrument used in a chain survey
- Fillet Weld
- Bulking of sand
- Bitumen concrete
Conclusion
Chlorination of water is a simple, cost-effective, and reliable method of ensuring safe drinking water. It plays a crucial role in preventing water-borne diseases by killing harmful pathogens such as bacteria, viruses, and protozoa. Apart from its primary function of disinfection, chlorination also improves water quality, taste, and odor, while providing residual protection during storage and distribution.
However, like any process, chlorination comes with its own set of challenges, such as the formation of harmful by-products, unpleasant taste, and limited effectiveness against certain microorganisms. Despite these disadvantages, its economical nature, easy application, and wide range of applications in municipal water supply, swimming pools, industrial systems, and emergency disinfection make it indispensable in modern water treatment.
By understanding the benefits and limitations of chlorination, we can ensure that it is used effectively and safely to provide clean, potable water to millions of people worldwide.
FAQs on Chlorination of Water
Q1. What is the chlorination of water?
Chlorination of water is the process of adding chlorine or chlorine compounds to water in order to kill harmful microorganisms and make it safe for drinking.
Q2. Why is chlorination important in water treatment?
Chlorination is important because it destroys disease-causing bacteria, viruses, and parasites, improves taste and odor, and ensures a safe water supply.
Q3. What are the main types of chlorination of water?
The main types are plain chlorination, pre-chlorination, post-chlorination, double chlorination, breakpoint chlorination, and super chlorination.
Q4. What are the advantages of chlorination of water?
It is cost-effective, easy to apply, reliable, and provides residual protection against recontamination.
Q5. What are the disadvantages of the chlorination of water?
Excess chlorine may cause bad taste and odor, form harmful by-products like trihalomethanes (THMs), and is less effective against some protozoa.
Q6. Where is chlorination of water commonly used?
It is commonly used in municipal water supply systems, drinking water treatment plants, and swimming pools.
Q7. What is breakpoint chlorination?
Breakpoint chlorination is the point at which the chlorine added to water completely reacts with impurities, and any further addition gives a free chlorine residual for disinfection.
Thank you 🙏 for spending your valuable time reading this article. We hope this guide helped you understand the Chlorination of Water simply and clearly. Keep learning, keep growing, and stay connected with Civil Weda for more exam-oriented and practical civil engineering content. 🚀