Introduction

Have you ever wondered why water boils at 100°C but alcohol seems to disappear even at room temperature? Or why do wet clothes dry faster on a hot, sunny day compared to a cold one?
All these everyday observations are connected to one invisible but powerful property of liquids, that is Vapour Pressure.
Every liquid around us has a natural tendency to change into vapour. Some do it quickly, some take time. The reason behind this difference lies in their vapour pressure, which controls how easily a liquid can evaporate or boil.
Understanding of vapour pressure is not only important in science but also in civil engineering, where it affects processes like concrete curing, drying of materials, and evaporation from water surfaces.
In this article, we will explore how vapour pressure works, the factors that affect it, and its significant role in both our daily lives and engineering applications. So let’s get started…
What is Vapour Pressure?
Every liquid has some molecules that continuously try to escape from its surface into the air. When these escaping molecules hit the walls of the container, they exert a certain pressure. This pressure created by the vapour molecules, when the liquid and vapour are in equilibrium, is known as Vapour Pressure. As per Wikipedia, it is also known as vapor pressure.
In simple words, a liquid tends to change into a vapour at a given temperature.
It tells us how easily a liquid can evaporate. The higher the vapour pressure, the faster the evaporation.
Imagine a closed container half-filled with water. Some water molecules escape into the space above and form vapour, while some of them return to the liquid. After some time, a balance is reached between the number of molecules leaving and returning. The pressure of this vapour at that stage is the vapor pressure of water at that temperature.
Factors Affecting Vapour Pressure
The vapour pressure of a liquid is not constant. It changes with temperature and the nature of the liquid. Some of the main factors that affect vapour pressure are discussed below.
1. Temperature
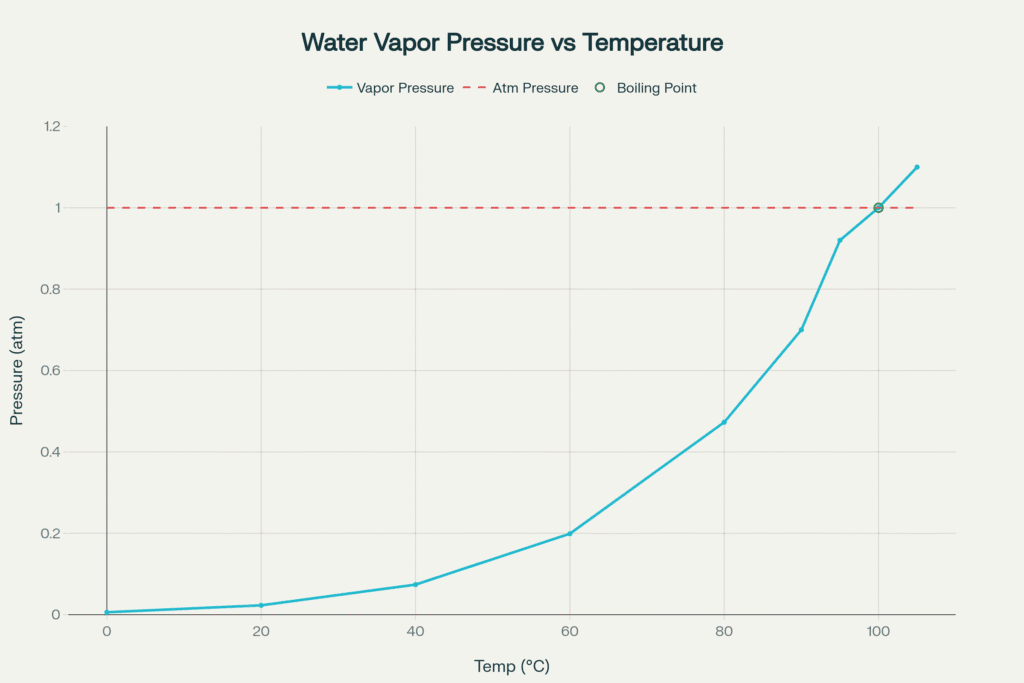
Temperature has the most significant effect on vapor pressure. When the temperature increases, the kinetic energy of liquid molecules also increases. As a result, more molecules can escape from the surface of the liquid into the vapour phase. Therefore, vapour pressure increases with an increase in temperature.
For example, at room temperature, water has a lower vapor pressure, but when heated, its vapour pressure rises until it equals atmospheric pressure. This is the point where the liquid starts boiling.
2. Nature of the Liquid
Different liquids have different tendencies to evaporate. Liquids with weaker molecular forces, such as alcohol or petrol, evaporate more easily and have higher vapour pressure. On the other hand, liquids like water or glycerin, which have strong intermolecular forces, show lower vapour pressure.
3. External Pressure
The external pressure acting on the surface of a liquid also affects its vapour pressure indirectly. If the external (atmospheric) pressure decreases, then the liquid will require less energy to reach its boiling point. This is why water boils at lower temperatures in high-altitude regions, where atmospheric pressure is lower than at sea level.
4. Nature of Impurities
The presence of impurities generally lowers the vapour pressure of a liquid. This happens because impurities reduce the number of surface molecules available for evaporation. For example, adding salt to water decreases its vapour pressure.
Relation Between Vapour Pressure and Boiling
Boiling is a familiar process that we see almost every day, but very few people know that it is directly controlled by vapour pressure.
A liquid starts boiling when its vapour pressure becomes equal to the surrounding atmospheric pressure. At this point, bubbles of vapour start forming inside the liquid and rise to the surface.
For example, at sea level, the atmospheric pressure is 1 atm (101.3 kPa). Water reaches this pressure at 100°C, so it boils at that temperature.
However, in high-altitude areas such as hilly or mountainous regions, atmospheric pressure is lower. As a result, the vapour pressure of water equals the atmospheric pressure at a lower temperature, so water boils earlier (for example, around 90°C in the mountains).
On the other hand, if the external pressure is increased (like in a pressure cooker), the liquid requires a higher temperature to reach the same vapour pressure. This is why food cooks faster in a pressure cooker, because water boils at a temperature above 100°C.
Real-Life Examples of Vapour Pressure
The concept of vapor pressure may sound theoretical, but we can easily observe its effects in our everyday lives. Here are some practical examples that help you understand it better.
1. Boiling of Water

When water is heated, its vapour pressure increases with temperature. At 100°C, the pressure of water becomes equal to atmospheric pressure, and boiling starts. At higher altitudes, atmospheric pressure is lower, so water boils at a temperature below 100°C.
2. Drying of Clothes
Wet clothes dry faster on a hot, windy day because high temperature and low humidity increase the vapour pressure. When the vapor pressure of water in the fabric becomes higher than that of the surrounding air, water molecules escape quickly, leading to faster drying.
3. Perfume and Alcohol Evaporation
Liquids such as perfume, spirit, or alcohol have high vapor pressure even at room temperature. That is why they evaporate easily and spread their smell quickly in the air.
4. Pressure Cooker
In a pressure cooker, the external pressure on the water surface increases. Due to this, water requires a higher temperature to reach the same vapor pressure as the surrounding pressure. Hence, the boiling point increases, and food cooks faster.
5. Evaporation in Civil Engineering Works
In civil engineering, this pressure plays an important role during concrete curing and surface drying. At high temperature and low humidity, the vapor pressure difference between the water in concrete and the surrounding air becomes large. This causes faster evaporation, which may lead to surface cracks if proper curing is not done.
Read more Civil Engg Topics
- Viscosity
- Portland Cement
- Bitumen Waterproofing
- Instruments used in a chain survey
- Bulking of sand
- Bitumen concrete
- Geopolymer Concrete
- Pile Foundation
- Surface tension
Conclusion
Vapor pressure is one of those invisible properties that quietly control many processes around us. From the boiling of water and drying of clothes to the curing of concrete, it plays a vital role in both daily life and engineering applications.
It depends mainly on the temperature and the nature of the liquid. When the temperature increases, molecules gain more energy and escape easily into the air, resulting in higher vapor pressure. Boiling starts only when the vapoor pressure of a liquid becomes equal to the surrounding atmospheric pressure.
For civil engineers, understanding vapor pressure helps in controlling evaporation rates, curing of concrete, and drying of materials, which are essential for maintaining quality and strength in construction works.
In simple words, it connects science with real-world engineering. By understanding it, we can better control processes that depend on heat, moisture, and evaporation.
FAQs
1. What is the vapour pressure of pure water?
It depends on the temperature. At room Temperature, the vapour pressure of pure water is approximately 3.17Kpa.
2. How is vapour pressure related to temperature?
Vapour pressure increases with an increase in temperature. i.e. directly proportional.
3. What happens to the vapour pressure when the external pressure increases?
At the same temperature, the vapour pressure remains the same.
Thank You for Reading! 🙏
We hope this article helped you clearly understand the Vapour Pressure in civil engineering. If you found this complete article useful, please share it with your friends and university students. For more informative posts on civil engineering topics, stay connected with Civil Weda. 🚀