Introduction

Have you ever noticed small water droplets sitting perfectly round on a leaf after rain? This happens because liquids naturally try to keep their surface area as small as possible. The property responsible for this behaviour is called surface energy.
In simple terms, this is the energy present on the surface of a liquid due to unbalanced molecular forces. It controls how liquids behave, form droplets, or spread on a surface.
In this article, we will learn what surface energy is, its definition, formula, and how it is related to surface tension, all explained in simple and easy-to-understand language for civil engineering students.
By the end of this article, you will start noticing how surface energies silently control many things around you. So let’s get started.
Definition of Surface Energy
Inside a liquid, molecules are surrounded by other molecules, so the forces on them are balanced. But the molecules at the surface have no neighbouring molecules above them, so they are pulled inward by the molecules below. Because of this unbalanced force, these surface molecules have extra energy stored in them, and this energy is known as surface energy.
So we can say that this energy is defined as the energy required to increase the surface area of a liquid by one unit.
In other words, it is the work done to create a new surface against the cohesive forces between the liquid molecules.
Molecular Explanation
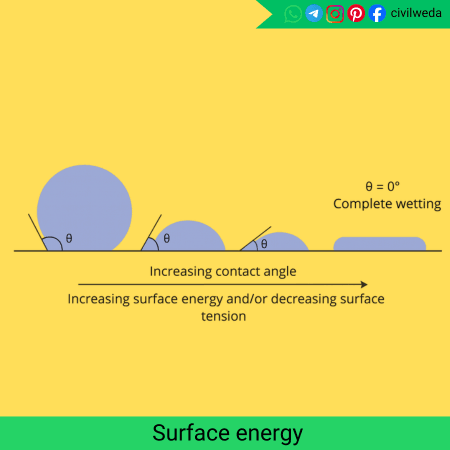
As we know, every liquid is made up of tiny molecules that attract each other through cohesive forces. Inside the liquid, each molecule is surrounded by other molecules on all sides. So the forces acting on it are balanced, which means no extra energy is stored there.
But the molecules present at the surface are in a different situation. They have neighbouring molecules only below and to the sides, not above them, so the forces on them are unbalanced.
As a result, these surface molecules are pulled inward by the molecules below, and this inward pull gives them extra potential energy.
This extra energy at the surface is what we call surface energy. That’s why a liquid surface behaves like a stretched elastic film. It always tries to stay as small as possible to reduce its energy.
Formula of Surface Energy
The formula for calculating energy is given by:
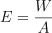
Where,
- E = Surface energy (J/m²)
- W = Work done to increase the surface area (Joule)
- A = Increase in surface area (m²)
It means that this energy is equal to the work done per unit increase in surface area. In simple words, when we stretch or expand the surface of a liquid, we need to do some work, and that work per unit area represents the surface energy.
Relation Between Surface Energy and Surface Tension
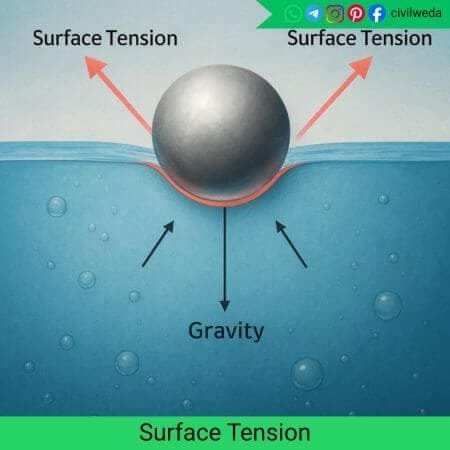
In liquids, surface energies and surface tension are closely related and are numerically equal. Both come in pictures due to the unbalanced molecular forces acting at the surface of the liquid.
When the surface of a liquid increases, some energy is needed to make it stretch or expand. The work done per unit increase in surface area is called surface energy, and the force acting per unit length of the surface is called surface tension.
Hence,
E = σ
Where,
- E = Surface energy (J/m²)
- σ = Surface tension (N/m)
Even though their units look different, 1 J/m² = 1 N/m,
So both are numerically equal, but mean slightly different things. It tells us about energy, and surface tension tells us about force.
Difference Between Surface Energy and Surface Tension
There are some important differences between surface energy and surface tension, which are tabled below:
| Property | Surface Energy | Surface Tension |
|---|---|---|
| Meaning | Energy required to increase surface area | Force acting along the surface per unit length |
| Symbol | E | σ |
| Unit | J/m² | N/m |
| Type | Energy term | Force term |
| Relation | E = σ | σ = E |
Real-Life Examples
Surface energy is not just a theoretical concept; it can be seen in many things around us. Here are a few simple examples that show how it works in real life:
Water droplets on a leaf
After rain, you might have seen small, round water droplets resting on a leaf. They form a spherical shape because the liquid tries to keep its surface area as small as possible to reduce surface energy.
Soap bubbles
When you blow a soap bubble, the film of soap solution stretches and stores energy. This energy is the surface energy, and it helps the bubble maintain its round shape for a short time.
Mercury droplets
Mercury does not wet the glass surface and stays in the form of small, shiny spheres. This happens because mercury has high energy, and it resists spreading. I have personally observed this behaviour, which perfectly proves how it works in real life.
Filling cracks with bitumen
In road construction, bitumen has strong surface energy and adhesion. That’s why it sticks well with aggregates and forms a waterproof layer.
Applications of Surface Energy in Civil Engineering
This energy plays an important role in many civil engineering materials and construction processes. Here are some common applications where this concept helps engineers understand material behaviour, which is
1. Paint and Coating Adhesion
2. Bitumen and Aggregate Bonding
3. Waterproofing Materials
4. Capillary Action in Soils and Concrete
5. Cleaning and Wetting of Surfaces, etc.
Read more Civil Engg Topics
- Viscosity
- Portland Cement
- Bitumen Waterproofing
- Instruments used in a chain survey
- Bulking of sand
- Bitumen concrete
- Geopolymer Concrete
- Pile Foundation
Conclusion
Surface energy may be an invisible concept, but its effects are visible everywhere around us, from a small raindrop sitting on a leaf to the waterproof coating on a concrete wall.
It explains why liquids form spherical shapes, why some materials repel water, and how bitumen or paint bonds strongly with a surface. For civil engineers, understanding energy helps in selecting better materials, improving adhesion, and designing more durable structures. In short, we can say surface energy is the hidden force that connects science with real-life construction. Once you understand it, you’ll start noticing its impact in almost every engineering material you use.
1. What is the SI unit of surface energy?
The SI unit is joule per square meter (J/m²)
2. What is the relation between surface energy and surface tension?
For liquids, surface energy (E) and surface tension (σ) are numerically equal: E = σ. It represents energy per unit area, while surface tension represents force per unit length.
3. What is the formula for surface energy?
The formula is: E = W / A
Thank You for Reading! 🙏
We hope this article helped you clearly understand Surface Energy in civil engineering. If you found this complete article useful, please share it with your friends and university students. For more informative posts on civil engineering topics, stay connected with Civil Weda. 🚀